Sputnik V recommended for emergency use
Sputnik V recommended for emergency use
Part of: GS Prelims and GS – II – Health
In news
- Russia’s COVID-19 vaccine — Sputnik V — has been recommended for emergency use authorisation in India following a meeting of the Subject Expert Committee (SEC).
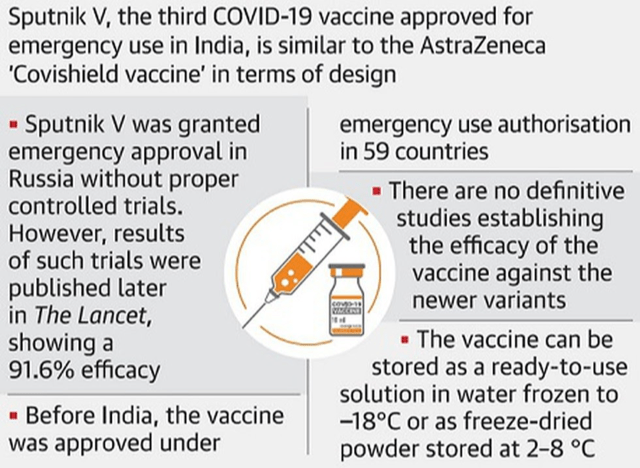
Key takeaways
- If approved by the Drug Controller General of India (DCGI), Sputnik V would be the third vaccine to be made available in India after the Serum Institute of India’s Covishield and Bharat Biotech’s Covaxin.
- Sputnik V is developed by Russia’s Gamaleya Research Institute of Epidemiology and Microbiology
- It claims to be one of the three vaccines in the world with efficacy of over 90%.
- The vaccine supplies for the global market will be produced by the Russian Direct Investment Fund (RDIF) international partners in India, Brazil, China, South Korea and other countries


Comments
Post a Comment